Resúmenes Epistemonikos
← vista completaPublicado el 13 de marzo de 2017 | http://doi.org/10.5867/medwave.2017.6863
¿Son efectivos los corticoides intratimpánicos en la enfermedad de Ménière?
Are intratympanic corticosteroids effective for Ménière’s disease?
Abstract
Ménière’s disease affects the inner ear and its main symptoms are vertigo, hearing loss and fluctuating aural symptoms. Nowadays, there are many therapeutic alternatives, being the use of intratympanic corticosteroids one that has become popular. To answer this question, we searched in Epistemonikos database, which is maintained by screening multiple databases. We identified four systematic reviews including 15 studies overall, of which seven were randomized trials. We extracted data and generated a summary of findings table using the GRADE approach. We concluded intratympanic corticosteroids probably do not decrease tinnitus, and might not decrease vertigo, hearing loss or aural fullness sensation in Ménière’s disease. Intratympanic corticosteroids probably do not cause important adverse effects.
Problem
Ménière’s disease affects the inner ear and it is characterized by episodes of recurrent vertigo, fluctuating sensorineural hearing loss and aural symptoms (such as tinnitus and aural fullness), being its main characteristic the fluctuation of its symptoms [1],[2]. The objective of the treatment is to reduce the intensity of the cardinal symptoms, to decrease the number of acute vertigo crises and to prevent the progression of the disease. Nowadays there are many therapeutic alternatives, including dietary sodium restriction, antivertiginous drugs, diuretics, intratympanic injection of drugs and even surgery. There is no consensus regarding the best intervention, nonetheless, one of the alternatives that has gained popularity in the last years is the injection of intratympanic corticosteroids [1].
Ménière’s disease is supposed to be an immunological disorder of the endolymphatic sac, with mechanisms involved at many levels. In this way, the injection of corticosteroids would have a role when absorbed towards the perilymph [1]. Its advantage would be achieving a higher concentration of corticosteroids in the inner ear, and avoiding adverse effects associated to systemic use. Also, it is a procedure that can be performed in an ambulatory setting [1]. However, there is a low risk of persistent tympanic perforation, dysgeusia, vertigo and pain; the latter two are generally self-limited [3].
Methods
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found four systematic reviews [1],[4],[5],[6] that include 15 primary studies reported in 17 references [7], [8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19], [20],[21], among them seven randomized controlled trials [7],[8],[10],[11],[12],[13],[14], from which four compare the intervention against placebo or no treatment, which correspond to the question of this summary [7],[8],[12],[13]. This table and the summary in general are based on the latter. |
|
What types of patients were included |
The four trials included patients with the diagnosis of Ménière’s disease with failure to medical treatment. The diagnostic criteria of the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) for the diagnosis of definite Ménière’s disease were used in two trials [8],[12]. None of the systematic reviews specified the criterion used for the remaining two trials [7],[12]. |
|
What types of interventions were included |
The trials assessed intratympanic injection of dexamethasone in different protocols:
Three trials compared against intratympanic injection of placebo [7],[8],[12] and one against medical treatment [13]. |
|
What types of outcomes |
The outcomes where grouped as follows:
|
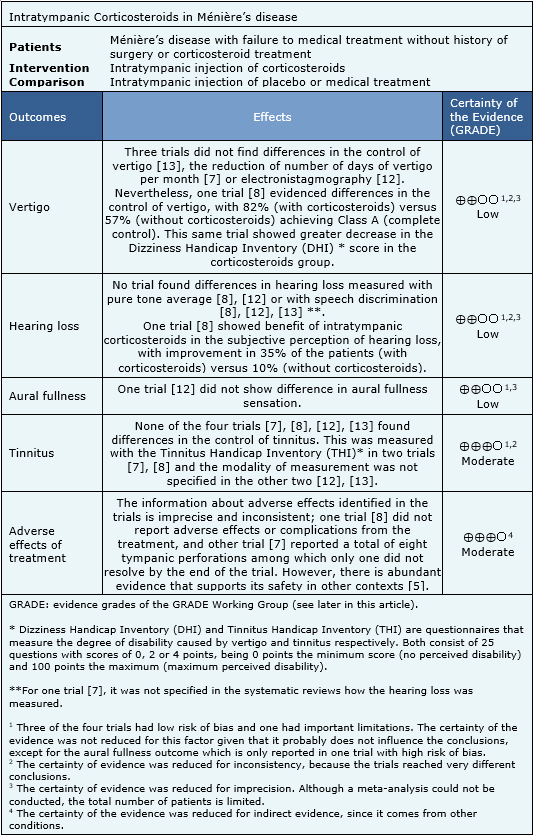
Summary of findings
The information regarding the effects of intratympanic corticosteroids in Ménière’s disease is based on four randomized trials that include 126 patients [7],[8], [12],[13]. All of the trials reported vertigo, hearing loss and tinnitus. Aural fullness was reported only by one trial [13] and the adverse effects were reported in two trials [7],[8].
The summary of findings is the following:
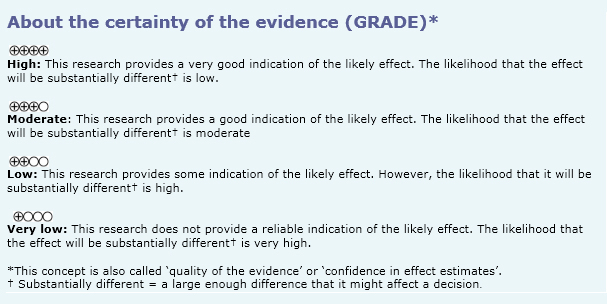
- Intratympanic corticosteroids might not decrease vertigo in Ménière’s disease, but the certainty of the evidence is low.
- Intratympanic corticosteroids might not decrease hearing loss in Ménière’s disease, but the certainty of the evidence is low.
- Intratympanic corticosteroids might not decrease aural fullness sensation in Ménière’s disease, but the certainty of the evidence is low.
- Intratympanic corticosteroids probably do not decrease tinnitus in Ménière’s disease. The certainty of the evidence is moderate.
- Intratympanic corticosteroids probably do not cause important adverse effects. The certainty of the evidence is moderate.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
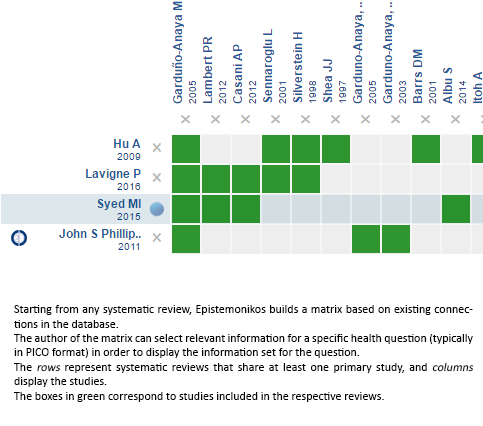
Follow the link to access the interactive version: Intratympanic steroids for Ménière's disease
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

