Resúmenes Epistemonikos
← vista completaPublicado el 22 de diciembre de 2017 | http://doi.org/10.5867/medwave.2017.09.7107
¿Es efectivo el uso de cannabinoides en el síndrome de emaciación (wasting) en VIH/SIDA?
Are cannabinoids effective for HIV wasting syndrome?
Abstract
INTRODUCTION Wasting syndrome is a common problem in HIV. It leads to substantive morbidity and mortality. The use of cannabinoids has been suggested as a treatment for weight, but it is not clear whether they are really safe and effective.
METHODS To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS We identified eight systematic reviews including ten studies overall, of which six were randomized trials. We concluded it is not clear whether cannabinoids increase appetite or weight in HIV wasting syndrome because the certainty of the evidence is very low, and they probably lead to frequent adverse effects.
Problem
The high frequency of HIV-related wasting syndrome, defined as the involuntary loss of at least 10% of standard body weight associated with chronic diarrhoea or chronic fatigue and fever for at least 30 days, was observed at the onset of the human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) epidemic [1]. This definition is no longer used, and the diagnosis is based mainly on the presence of significant, progressive or very fast weight-loss, which has led to various new definitions [2].
After the beginning of anti-retroviral therapy an important decrease of wasting syndrome cases was observed, but it still remains a common problem, estimated to affect up to 14-38% of patients [3].
Its relevance lies in its association to a greater risk of mortality [3] which makes important to look for treatment alternatives aimed to optimise the nutritional state [2].
It has been suggested the use of natural or synthetic cannabinoids would have a positive effect on appetite, weight gain and mood of adults with HIV/AIDS [4]. They would increase appetite through activation of CB1 endocannabinoid receptors at a central level. However, the real clinical effect of this treatment is still unclear [5].
Methods
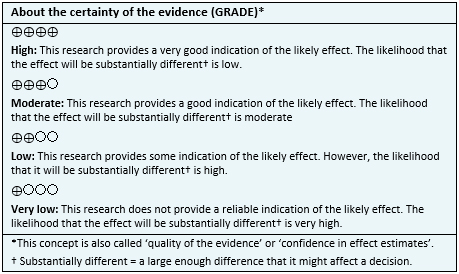
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found eight systematic reviews [4],[6],[7],[8],[9],[10],[11],[12] which include ten primary studies [13],[14],[15],[16],[17],[18],[19],[20],[21],[22] of which six are randomized controlled trials [13],[14],[15],[16],[17],[18]. This table and the summary in general are based on the latter. |
|
What types of patients were included* |
All of the trials were conducted in adults who were predominantly male. Average age ranged between 36 and 43 in the different trials. Intervention duration ranged between 2 weeks and 12 months. One of the trials was conducted in hospitalized patients [18], and the rest in outpatients. In five trials [13],[15],[16],[17],[18] certification of HIV infection was required in order to participate, and one of them included AIDS-related criteria [14]. In five trials [14],[15],[16],[17],[18] an ongoing antiretroviral treatment was required. The trials did not mention CD4 count and only one makes reference to viral count [16]. Four trials [13],[14],[15],[17] included some criteria related to weight-loss within a certain interval of time. Regarding previous cannabinoid use, two trials [17],[18] included patients that were cannabis consumers, while the other four required a given interval without cannabinoid use prior to participation [13],[14],[15],[16]. |
|
What types of interventions were included* |
All of the trials evaluated the effect of dronabinol in various doses, administered orally between one and four times a day. In three trials [16],[17],[18] dronabinol was compared to inhaled marijuana in the form of cigarette with different doses of THC (between 1,8% and 3,9% THC content) and placebo. In one trial dronabinol was compared to megestrol [15], a steroid, without comparing with a placebo. Five trials compared against placebo [13],[14],[16],[17],[18]. |
|
What types of outcomes |
Appetite increase was evaluated in all of the trials with different visual analogous scales, along with adverse effects. Five trials [13],[14],[15],[16], [18] evaluated weight gain expressed as total weight gain [kg] in a given interval. In addition, functionality scales, subjective experience and mood changes were also evaluated. Only one trial evaluated laboratory nutritional indicators [16]. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
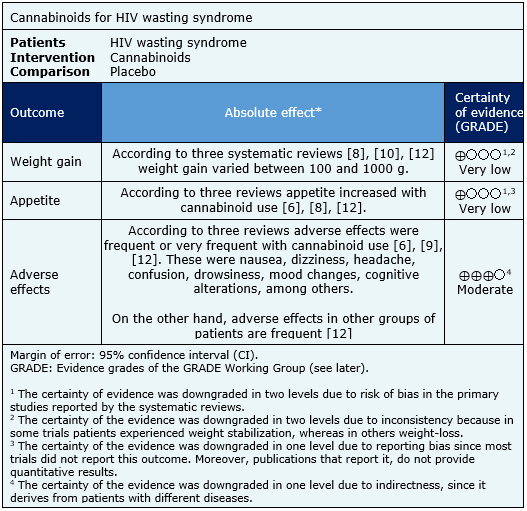
Summary of Findings
The information on the effects of cannabinoid use in HIV wasting syndrome is based on six randomized trials [13],[14],[15],[16],[17],[18] which included 298 patients.
All of the trials reported change in appetite (298 patients) and adverse effects (298 patients). Five trials [13],[14],[15],[16],[18] measured change in weight (268 patients).
However, none of the reviews identified was able to pool data from the trials into a meta-analysis, so the results are presented in a narrative form, based on the conclusions of the individual reviews.
The summary of findings is the following:
- It is not clear whether the use of cannabinoids in HIV wasting syndrome leads to weight gain because the certainty of the evidence is very low.
- It is not clear whether the use of cannabinoids in HIV wasting syndrome leads to an increase in appetite because the certainty of the evidence is very low.
- The use of cannabinoids in HIV wasting syndrome is probably associated to frequent adverse effects.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
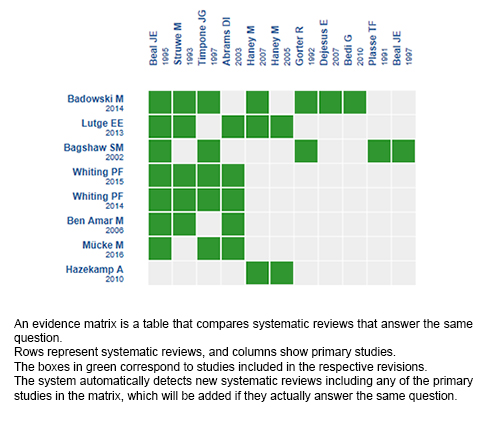
Follow the link to access the interactive version: Cannabinoids for HIV/AIDS wasting syndrome
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.

