Análisis
← vista completaPublicado el 2 de junio de 2022 | http://doi.org/10.5867/medwave.2022.05.2560
Vigilancia epidemiológica en pandemia de COVID-19: Sistema EPIVIGILA
Epidemiological surveillance in COVID- 19 pandemic: EPIVIGILA system
Abstract
In March 2020, the first version of EPIVIGILA was deployed in a productive environment a few days after the first local case of COVID- 19. This system is a technological integration plat-form for national epidemiological surveillance of notifiable diseases. Previously, Chile used a manual process that would probably have failed with a peak volume of more than 38 000 daily notifications; in a country with 18 million inhabitants, long and narrow geography, and centralized governance. This work highlights the importance of the national electronic surveillance system – EPIVIGILA – in managing the pandemic. The system’s main strength is its ability to adapt to the needs of reliable, precise, timely, and real- time information. EPIVIGILA was able to include, under the circumstances, different flows, actors, data, and functionalities with high expectations of accuracy. This valuable information allowed the authorities to assess the impact of the measures to manage and control the pandemic. Its versatility positions this platform among the few globally that operates national data with a high level of granularity in a single system through a pandemic. In Chile, EPIVIGILA is the primary source of information for daily reports, epidemiological reports, and data published on government websites about COVID- 19. Thus, electronic systems prove fundamental for public health because the recording and processing of data generate clear, reliable, and timely information, helping authorities make decisions to reduce the spread of infectious diseases, prevent deaths, and improve the population’s quality of life.
|
Main messages
|
Introduction
Historically, many epidemiological events have threatened humanity, including smallpox, cholera, the SARS epidemic, the influenza A (H1N1) pandemic, the Zika virus, and currently, the SARS- CoV- 2 pandemic [1]. In these scenarios, epidemiological surveillance constitutes an essential function in public health [2], defined as a continuous and systematic process of collecting, analyzing, and interpreting health data to describe and monitor a health event to support the planning, implementation, and evaluation of public health interventions and pro-grams [3]. In other words, surveillance is designed and implemented to provide valid, reliable, and timely information for the health authority, recognizing that it supports actions aimed at achieving highly relevant solutions [4].
Epidemiological surveillance aims to early detect and timely manage people who require health services and establish the population’s health status to manage disease prevention and control measures [5]. An essential challenge for epidemiological surveillance systems arises in epidemics, endemics, and pandemics, where diseases must be reported immediately to timely control cases and develop follow- up measures [6]. In this regard, some authors point out that "given that SARS- CoV- 2 is likely to become endemic in many countries [...] a technological platform for epidemiological surveillance may represent a fundamental tool" [7]. However, manual notification has been used for a long time in Latin American countries, relegating its use to complement digital and technological resources with different degrees of development. Analyzing this aspect is relevant to assessing health authority’s possibilities with manual or mixed modalities to control infection outbreaks and making appropriate and immediate decisions in the face of thousands of new daily cases that those responsible must notify. In such a scenario, manual modality is likely to fail because it lacks a precise, fast, reliable, and automated process, affecting the flow of information and seriously impacting the loss of human lives.
Since 2014 and until the beginning of 2020, the surveillance of notifiable diseases in Chile was regulated by Decree 158 [8].
Then, the surveillance was replaced by Decree 7 [6] and Ordinary 845 [9]. The latter establishes that notifications must be made by an accredited user (physician) by completing the corresponding form, available on the integrated national surveillance platform EPIVIGILA by RAVENO (from now on EPIVIGILA).
This paper highlights the importance of the national electronic surveillance system – EPIVIGILA – in managing the COVID- 19 pandemic. For the first time in Chile, this system allowed the health authority to have at its disposal an enormous amount of structured and quality data that supported decision- making, planning, and evaluation of informed health strategies.
Discussion
Surveillance systems are built to detect and circulate information relating to the occurrence and distribution of diseases [6] prioritized according to international agreements and regulations of each country. The selected diseases represent a risk for the population due to their epidemic potential and should be addressed according to health authorities' regulations [3],[6],[9],[10],[11]. In Chile, the surveillance of communicable diseases is based on Decrees 725 [10] and 7 [6]. These regulations state that the SARS- CoV- 2 disease is "notifiable because it is an unusual or unforeseen disease of infectious origin. Therefore, any suspected case must be reported immediately by the treating physician" [12].
The EPIVIGILA surveillance system was implemented in 2019 and deployed in a productive environment in March 2020 to become an agile, dynamic, immediate, modern, and secure strategy for collecting national health information for notifiable diseases. Until then, notification was done through a paper form issued by the health professional and then entered into the respective regional offices [11],[13]. In response to the COVID- 19 epidemic, the EPIVIGILA platform incorporated a new notification module for this disease. Thus, the enormous capacity to incorporate data is a crucial feature of this tool that allows authorities to evaluate the impact of public health interventions.
The COVID- 19 reporting process in EPIVIGILA (Figure 1) starts with a physician consultation. This case may be notified with a suspicion status and request a reverse transcription- polymerase chain reaction (RT- PCR) test depending on the cur-rent clinical criteria. Until the specimen result, the notification will be maintained as a suspicious case. Upon receiving the test result, the case may be confirmed or stated as probable (when the sample is insufficient to confirm or rule out the disease). The change in status (including suspicion) initiates isolation and follow- up actions triggered upon receiving a positive laboratory sample not yet reported in EPIVIGILA. When the sample is negative, the notification is discarded, and it is not necessary to maintain patient follow- up.
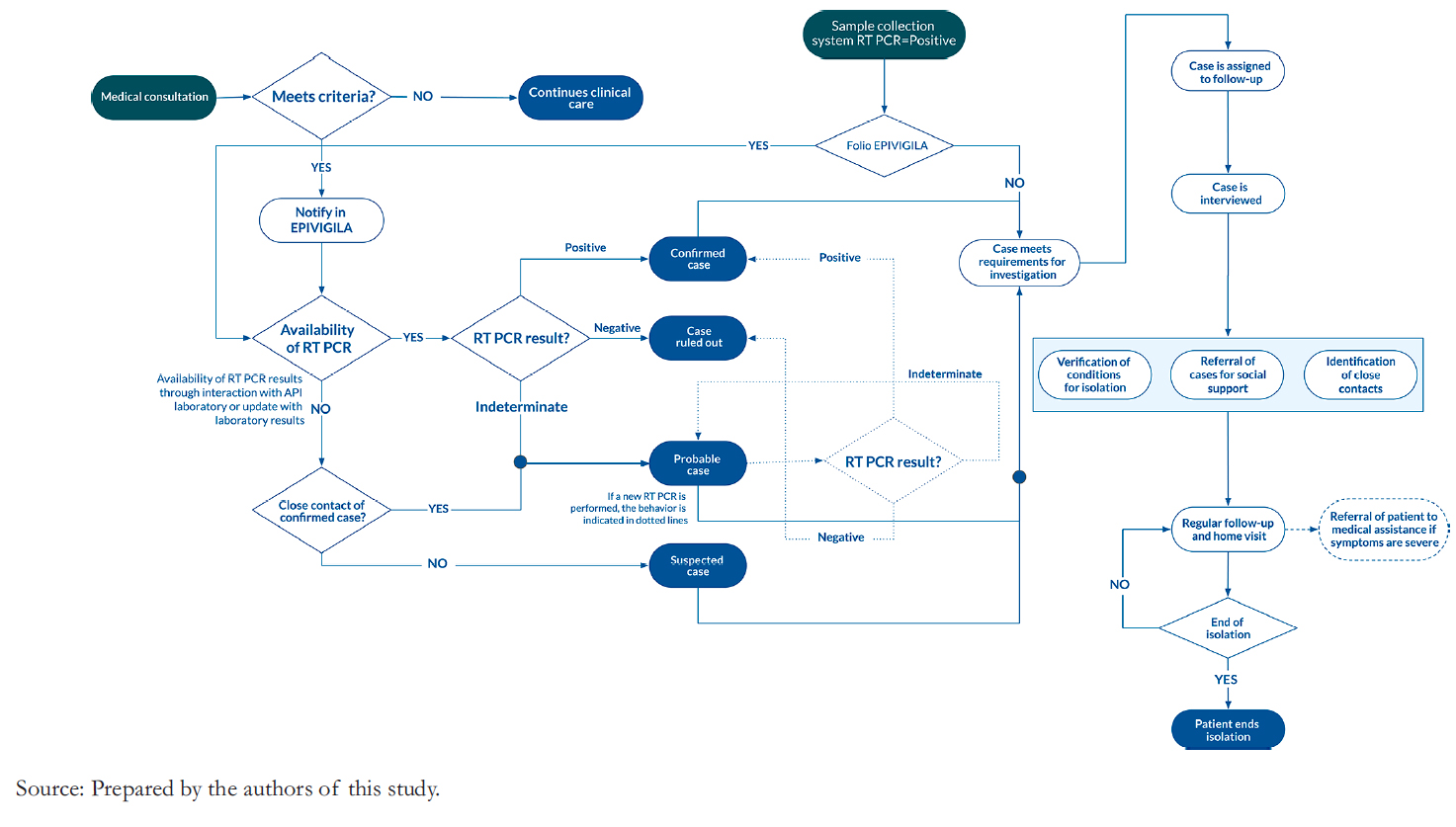 Full size
Full size Case notification is sent to the provincial services, where the data are validated and continue to the regional services. The reported cases are validated and forwarded to the central authorities, who prepare the epidemiological reports and use them for public health decisions [11]. The system is integrated with the sampling system, and physicians directly make the notification of cases in public and private institutions. The system receives the notification of the tests applied and RT- PCR results delivered by more than 200 accredited laboratories (including 40 public hospitals, 32 private laboratories, and 28 universities) [14].
Regarding surveillance in Chile, experts from the Pan American Health Organization and the World Health Organization (WHO) recognized "Chile’s historical trajectory in disease surveillance and information management" [15]. Likewise, it is highlighted that the reports consider more than 80% of the main variables. Although epidemiological surveillance in Chile had been effectively developed manually for decades [14], this process did not take advantage of the capabilities of new technologies, which offer shorter reporting times for physicians.
Moreover, the EPIVIGILA system improved the epidemiological surveillance of COVID- 19 by recording data from the primary source (the physician and laboratories) and epidemiological surveillance with quality and timely data to support control, follow- up, traceability of cases and contacts, and detection and early warning of outbreaks.
One way to measure the usefulness of the surveillance system is to observe how consistent it is with the criteria assumed by the health authority for these tools – i.e., by allowing a continuous and systematic collection, analysis, and interpretation of health data to describe and monitor a health event. A surveil-lance system should contribute to the planning, execution, and evaluation of public health interventions [16]. In EPIVIGLA, we can recognize a total concordance with the criteria above. The system systematically and continuously complements the epidemiological surveillance process, collecting detailed data, including the minimum data suggested by WHO [16] for surveillance, as detailed in Table 1.
 Full size
Full size Table 1 shows that the processing of the data stored in EPIVIGILA allows delivering detailed and diverse information. This platform is among the few globally operating with high granularity data integrated into a single national registry system in a pandemic. This system requires fine granularity concerning the data it stores because the higher the level of detail, the greater the analytical possibilities. The data can be summarized or aggregated, making it a fundamental tool for supporting public health decision- making in the country. This information permits evaluating the effects of strategies and making corrections according to the evolution of the pandemic [17],[18].
It should be reiterated that this system was not explicitly created for the registration of COVID- 19 cases but all notifiable dis-eases. Before 2020, the notification of diseases averaged 50 cases per day, which the manual system could cover. The escalation of notifications in such a short time is relevant to see the team’s burden and capacity to adapt and respond to the large volume of daily notifications that the health system had to assume from the beginning of the COVID- 19 pandemic and during the most chaotic months between May and June 2020. During this period, figures exceeding 6000 confirmed cases per day were reached, as occurred on June 7 (6405), 12 (6754), 13 (6509), 14 (6938), and 19 (6290) [18]. On April 8, 2021, a new record of 9171 new cases per day was reached [17]. Then, COVID- 19 infections exceeded 35 000 new cases per day early in 2022, as seen on February 3 (35 197), 4 (37 468), 5 (36 297), and 11 (38 446) [19]. Chile was one of the most affected countries in the first half of the pandemic, ranking sixth in terms of the number of infected people among more than 200 countries worldwide [20].
It is essential to highlight data protection throughout the processing chain in the EPIVIGILA platform. First of all, only users with a unique, encrypted key and password have access. Users have different roles that allow access to information depending on their permissions. The data is supervised by the platform’s management and development team, the central health authority, and the Ministry of the Interior authorities and is subject to a security audit every two months. In addition to the above, the system is on Amazon, so it follows strict security measures provided by the infrastructure.
Another way of measuring the support of this technological system for national health notification and surveillance is by analyzing the percentage of access errors, where higher errors mean a lower availability. These errors imply that the tool does not respond to the universe of users and requests it receives. In this case, EPIVIGILA presented an annual error rate of 0.18% (2021), which means a 99.82% average availability for that year.
According to the above, EPIVIGILA provides national authorities with a system that has enabled a continuous and systematic collection and analysis process, carried out in real- time, with different validation methods and a minimum set of consensual reporting data. This system facilitates the Chilean population’s management, traceability, prevention, and control of events. From the stored data, the EPIVIGILA system provides reports in tables, graphs, and maps that facilitate surveillance and the necessary actions for the management and control of the pan-demic, constituting a technological system that supports health authorities in decision- making and potentially improving the health of the population. Precisely, in the COVID- 19 pan-demic, this corresponds to one of the primary sources of information for the preparation of official epidemiological reports, for the daily reports delivered to the public opinion and on different governmental websites (Ministry of Health; Ministry of Science, Technology, Knowledge, and Innovation; Ministry of National Assets, among others). In addition to the above, as of July 2020, the data entered into the platform will generate information for the traceability and isolation testing process. Specifically, this functionality was added for this pandemic, allowing the completion of diverse information to ensure the isolation of cases, quarantine for contacts, and organize the respective compliance schedules, among other features. Thus, it is possible to visualize the process by different users who have different access to the information, depending on their roles.
Finally, the impact of the EPIVIGILA platform can also be measured considering the high use of the data hosted on the sites mentioned above (plus several associated social networks). These data have served in the scientific work, being a source for many researchers who generated more than a hundred products (dashboard, predictive models, viewers, scientific publications, among others) [21]. Consequently, the above shows that this tool has enabled a large and diverse amount of data used in different fields.
Final considerations
The COVID- 19 pandemic has been a challenging experience worldwide. New technologies are at the service of solutions, proving vital support in public health. They provide secure, agile, digitized information systems that rapidly collect data and generate reports and alerts in the shortest possible time. In this sense, EPIVIGILA has supported public health control in Chile.
It is important to consider that this system results from awarding projects with competitive state funds, in charge of researchers from regional and state universities. They generated a tool with a tremendous visionary approach, anticipating when nothing foresaw such a chaotic scene as the COVID- 19 pandemic.
Thus, State agencies were provided with a tool for epidemiological surveillance that favors the national population’s health, survival, and quality of life.
However, EPIVIGILIA is an ongoing challenge because the system’s usefulness is constantly being tested, as new diseases or the re- emergence of others are unpredictable. When these outbreaks occur, they do not have a priori known patterns, so technological surveillance, managers, and providers are demanded to collaborate and permanently learn from emergencies, outbreaks, epidemics, or pandemics.
In short, as a living system, this instrument requires a recursive, progressive, and constant learning process. In addition, international networks and alliances are still pending, which would strengthen these tools.

