Revisión clínica
← vista completaPublicado el 11 de diciembre de 2021 | http://doi.org/10.5867/medwave.2021.11.002132
Troponinas cardíacas: información actual sobre las principales características analíticas de los métodos para la determinación y nuevas posibilidades diagnósticas
Cardiac troponins: current information on the main analytical characteristics of determination methods and new diagnostic possibilities
Abstract
The methods used to diagnose cardiovascular diseases are constantly being improved, leading to an expanded perception of the diagnostic value of biomarkers and their new diagnostic possibilities. One striking example are the main biomarkers used to diagnose acute myocardial infarction: cardiac troponins. The first methods for determining cardiac troponins (proposed 30 years ago) were characterized by extremely low sensitivity. Therefore, they could only detect large acute myocardial infarctions. These methods were also characterized by low specificity, expressed as a high probability of cross-reactivity with skeletal troponin isoforms, which generated false-positive results in the presence of skeletal myopathies and skeletal muscle lesions. With the introduction of high-sensitivity cardiac troponins into clinical practice, the possibility of early diagnosis and exclusion of acute myocardial infarction by assessing troponin concentrations in the first few hours (from admission to the first hour, second hour, or third hour) has become more specific. Our knowledge about cardiac troponins has changed over the years and promising new medical uses have emerged. This paper reviews current data on the diagnostic value of cardiac troponins, the main methods used for their determination, and their analytical characteristics from historical and modern insights.
|
Main messages
|
Introduction
The troponin complex of striated heart muscle tissue consists of three proteins: troponin I (cTnI), troponin T (cTnT), troponin C (cTnC); which together with tropomyosin, play a vital role in regulating contraction and relaxation of heart muscle [1]. The amino acid structure of the troponin complex proteins is essential for maintaining function. According to genetic studies, many mutations exist within genes encoding protein molecules of the troponin complex. These mutations cause severe and potentially fatal inherited cardiac function disorders known as cardiomyopathies [2]. In two myocardial troponin complex proteins (troponin I and troponin T), the amino acid structure differs from their skeletal muscle isoform. This difference makes them unique and allows them to be used as biomarkers to detect ischemic myocardial damage in acute myocardial infarction. The amino acid structure of troponin C is identical in cardiac and skeletal muscle tissues, and therefore it has low specificity to diagnose acute myocardial infarction. Although troponin I and T are thought to be localized only in the myocardium, some investigators have found extramyocardial expression of these troponins in skeletal muscle [3],[4],[5] and in the muscle membrane of the vena cava wall and pulmonary veins in humans and other mammals [6]. Therefore, troponin I and T cannot be considered exclusive cardiac markers. Little is known about the causes and mechanisms underlying extramyocardial expression of cardiac troponins, so further research is needed.
Cardiac muscle contains approximately four to six milligrams of troponin I and 10 to 11 milligrams of troponin T in one gram. Approximately 95% of the troponin complex (structural troponin fraction) participates in myocardial contraction. Instead, the remaining 5% of troponin I and T molecules are located in the cytosol of myocardial cells (cytoplasmic fraction of troponins) and do not regulate cardiac muscle activity [7].
Diagnostic value and mechanisms increasing serum cardiac troponins
Among biomarkers proposed for diagnosing acute myocardial infarction, troponins I and T are the most reliable and most used in clinical practice. However, they differ from an ideal biomarker because they are elevated relatively late from myocardial ischemia (pain syndrome) and do not have absolute specificity for detecting cardiac ischemic necrosis. Incorrect diagnoses may be present when troponin I and T levels are significantly increased by other (non-ischemic) myocardial lesions, unrelated to acute myocardial infarction [8],[9],[10],[11],[12],[13]. Given this evidence, a more competent and modern definition of the diagnostic value of troponin I and T could be as follows: "troponin I and troponin T are specific cardiac markers of cardiac damage, although they are not specific of any particular type of damage – including ischemic necrosis of myocardial cells in acute myocardial infarction". Therefore, physicians should not rely solely on laboratory diagnostic methods (i.e., troponin I and/or troponin T) during the therapeutic and diagnostic process when admitting patients with signs of acute myocardial infarction.
We should note that the dynamics of troponin I and T increase can be similar in the early stages of many diseases causing irreversible (e.g., acute myocardial infarction, myocarditis) or reversible (e.g., physical exertion, stress conditions) myocardial damage. The latter aspect creates significant difficulties in establishing a diagnosis [12],[14]. Researchers have not yet established the exact nature of all cardiomyocyte diseases, as mechanisms underlying this damage are very complex. For example, in systemic inflammation (sepsis), circulating cytokines in the blood directly damage myocardial cells and indirectly affect cardiac tissue, increasing myocardial oxygen demand. This pathological state generates myocardial ischemia with intact coronary arteries, corresponding to a type 2 acute myocardial infarction [15].
Several factors also affect troponins I and T increase in chronic renal failure. According to some studies, troponins increase in chronic renal failure is due to a decreased blood clearance rate into the urine. Among patients with reduced glomerular filtration rate, serum troponin T levels increase significantly more [16]. It has been suggested that in patients with chronic renal failure, cardiomyocyte damage and increased serum troponin I and T levels are caused by a direct effect of metabolic wasting products, in particular products of nitrogen metabolism. It was also hypothesized that in chronic renal failure, the expression of troponins I and T of skeletal muscles is activated, which may be another underlying mechanism behind troponins increase [4],[12],[16].
Reversible damage of myocardial cells under certain physiological conditions (physical activity during prolonged running or severe stressful situations) and pathological conditions (such as transient ischemic episodes in angina pectoris) slightly increase troponins I and T – which usually does not exceed 5 to 10 times the initial values. This small increase of troponins I and T suggest that only troponins' cytoplasmic fraction is released to circulation. On the other hand, higher plasmatic levels of troponins I and T are caused by the damage of the structural fraction of the cardiomyocyte contractile apparatus, which is typical of irreversible myocardial lesions.
High-sensitivity immunoassays: how has ouR undeRstanding of the biochemistRy and diagnostic value of troponins changed?
The creation of high-sensitive immunoassays for the detection of troponin I and troponin T (hs-cTnI and hs-cTnT), improved diagnostic capabilities, and promising directions for future research have been significantly expanded [1],[8],[12]. In particular, the possibility emerged to determine lower concentrations of troponin I and T molecules that had previously remained invisible to moderately sensitive immunoassays.
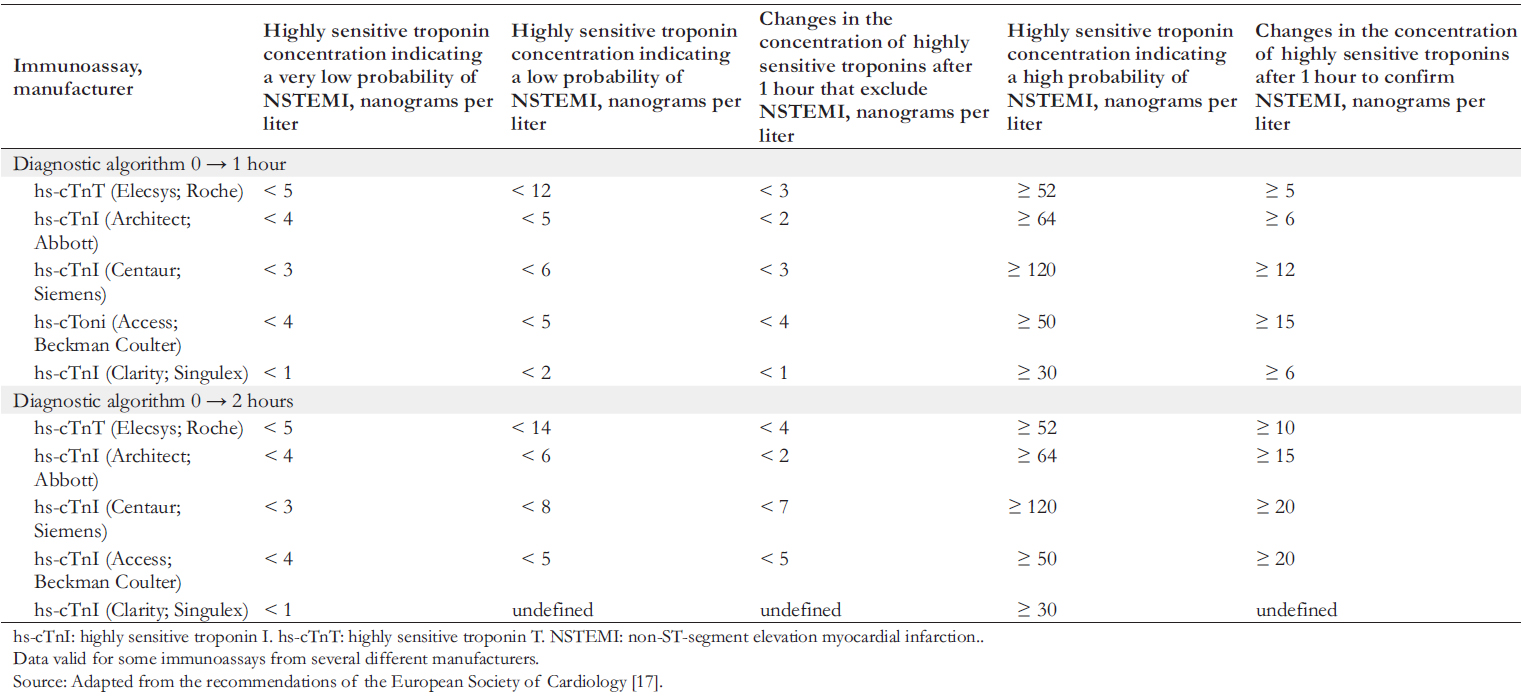
This advance has allowed the development of early diagnostic algorithms for the confirmation or exclusion of acute myocardial infarction. The European Society of Cardiology emphasizes the high efficiency in using early algorithms (0 → 1 h and 0 → 2 h) to exclude and confirm non-ST-segment elevation myocardial infarction [17]. These diagnostic algorithms for early diagnosis of non-ST-segment elevation myocardial infarction are based on assessing highly sensitive cardiac troponin levels on admission and then re-determining concentrations after one or two hours. Thresholds of highly sensitive cardiac troponins for confirming or excluding non-ST-segment elevation myocardial infarction are specific to immunoassays from different manufacturers (Table 1).
If the concentration of highly sensitive cardiac troponins measured on admission is very low or low, and there is no significant increase after one (or two) hours (Table 1), the diagnosis of non-ST-segment elevation myocardial infarction is excluded. In these cases, early discharge and outpatient treatment are recommended. If the concentration of highly sensitive cardiac troponins measured on admission is above the thresholds or shows a significant increase after one or two hours, there is a high probability of non-ST-segment elevation myocardial infarction. Such patients are recommended for hospitalization and invasive coronary angiography for emergency cases.
 Full size
Full size In addition, increased sensitivity of these methods allowed predicting levels of high-sensitivity cardiac troponins I and T in many pathologies causing myocardial cell damage [12]. The 99th percentile (serum troponin levels above 99% of healthy individuals) was proposed as the upper reference value to indicate an unfavorable prognosis.
Highly sensitive methods for determining troponin I and troponin T have changed concepts about biochemical characteristics. It has been shown that serum troponin levels in healthy patients depend on sex, age, and daytime of the biomaterial acquisition [18],[19],[20],[21],[22]. Based on this, it has been proposed to adjust the 99th percentile to some of these factors.
The effect of sex on troponin levels proved to be highly significant and is considered in new rapid algorithms for diagnosing acute myocardial infarction [1],[22],[23]. It has been suggested that differences in troponin values based on sex (higher levels of high-sensitivity cardiac troponin I and T in males) are explained by a more prominent left ventricular myocardial mass in males than females [22]. Likewise, age alters high-sensitivity cardiac troponin I and T levels. In younger healthy patients, serum troponin levels are significantly lower than in older patients. These age-associated changes are suggested to be caused by chronic (latent) comorbid pathologies in the elderly that may adversely affect myocardial cells and promote an increased release of troponin I and T molecules [23],[24].
Furthermore, there are reports of circadian rhythms in highsensitivity cardiac troponins I and T (i.e., the dependence of daytime on the concentration of cardiac troponins). It has been shown that serum levels of high-sensitivity cardiac troponins I and T in patients are significantly higher in the morning than in the evening [18]. This trend is described for healthy patients [18] and patients with chronic renal failure [25].
The mechanisms that explain the circadian rhythms of highsensitivity cardiac troponin I and T are not well known; however, there is speculation about their association with other circadian rhythms that may negatively affect myocardial cells. For example, the sympathetic and renin-angiotensin-aldosterone systems have maximal activity in the morning, leading to increased cardiac muscle load, heart rate, and blood pressure. The impact of this system on the myocardium is similar to physiological changes occurring during exercise and stress, which are accompanied by an increase of high-sensitivity cardiac troponins I and T. It is believed that the increased activity during the morning period was an evolutionary advantage to ensure wakefulness. However, these systems also play vital roles in the pathogenesis of cardiovascular diseases, including acute myocardial infarction. Consequently, they may be additional heart damage in patients with cardiovascular risk factors (e.g., atherosclerosis, dyslipidemia, hemostatic disorders) [26].
It is worth noting that the influence of age and daytime on high-sensitivity cardiac troponins I and T is debatable due to insufficient evidence and conflicting research results. Therefore, in modern diagnostic algorithms of acute myocardial infarction, the influence of these factors is still not considered. To exemplify this controversy, van der Linden [25] reported a pronounced effect of circadian rhythms over high-sensitivity cardiac troponin T on early diagnosis of myocardial infarction. On the other hand, Klinkenberg [18] found that circadian rhythms of high-sensitivity cardiac troponin T have no significant effect on early diagnostic algorithms. Concerning the design of these studies – contradictory in terms of the clinical significance of circadian rhythms of high-sensitivity cardiac troponin T – some differences in the clinical characteristics of patients are observed. Particularly, the degree of diurnal fluctuations in high-sensitivity cardiac troponin T levels in the van der Linden study appears to have been influenced by chronic renal failure.
Investigating cardiac troponins in noninvasively produced body fluids
The primary biological fluid for determining troponins is the blood. However, with the advent of highly sensitive methods, it became possible to determine troponins in other biological fluids that can be obtained noninvasively. Obtaining this biomaterial from patients is atraumatic, painless, and reduces the risk of developing blood-borne infections (e.g., human immunodeficiency virus and viral hepatitis). Additionally, it does not require trained medical personnel and permits the patient himself to take his biomaterial at home. For example, troponin concentrations in urine are small and therefore not detected by moderately sensitive test systems. However, highly sensitive screening methods detected troponins in the morning urine of all screened individuals. High-sensitive cardiac troponin T levels in the urine of patients with hypertension were significantly higher than in patients with normal blood pressure [27],[28].
Oral fluid is another promising noninvasive biomaterial for diagnosing many endocrine, oncologic, and cardiovascular diseases, including acute myocardial infarction [29],[30],[31],[32]. Our singlecenter pilot study recently demonstrated that the concentration of high-sensitivity cardiac troponin I in oral fluid of patients with acute myocardial infarction is significantly higher than controls. In addition, high-sensitivity cardiac troponin I levels in blood serum and oral fluid had a moderate correlation [31]. Further studies on larger samples are needed to establish reference values and standardize the preanalytical stage to increase the clinical and diagnostic value of high-sensitivity cardiac troponin I in oral fluid.
Cardiac troponin determination methods: a brief history of the development of troponin immunoassays
Determination of troponins in the blood is carried out through different immunochemical methods (e.g., radioimmunoassay, enzyme immunoassay, immunofluorescence analysis, and immunochemiluminescence). This process includes several successive stages: immunological, chemical, and detection.
Within the first stage (immunological), there is a specific interaction of diagnostic antibodies of a commercial kit with an antigen, which (in this case) is troponin. In the second and third stages, there is either an additional immunological reaction of antibodies and the formation of a sandwich complex or a chemical (enzymatic) reaction and recording of the received signal. Signal detection methods differ depending on the antibody marker used. Through enzyme immunoassay, the color intensity is evaluated using a photometer/spectrophotometer. Radioisotopes (radionuclides) are used as markers in radioimmunoassays and are evaluated with a radiometer (radio spectrometer). regarding fluorophores, the signal is recorded in a fluorometer. The signal strength is directly proportional to the concentration of troponins in the biological sample. The results are often expressed in quantitative values (nanograms per milliliter, nanograms per liter, micrograms per liter) or through a visual assessment of the number of strips formed, typical of qualitative methods (diagnostic test strips) used at the patient’s bedside.
There is a long-gone need to create specific immunochemical assays to determine troponin I and troponin T for diagnosing acute myocardial infarction. In 1987, Cummins reported the first method (first-generation immunoassays) to determine troponin I. This method had a high minimum determinable concentration (about 10 micrograms per liter or 10,000 nanograms per liter). The troponin increase was observed only in largescale acute myocardial infarction and at late dates from patient admission. Therefore, this immunoassay was not suitable for practical medicine and was significantly inferior to the diagnostic value of creatine kinase MB, which at that time was generally considered the gold standard for diagnosing acute myocardial infarction [33]. In the following years, a research team led by Katus developed an enzyme-linked immunosorbent assay to determine troponin T. The minimum detectable concentration in this method improved to 100 nanograms per liter, and the laboratory test time took 90 minutes [34]. According to a study using this immunoassay, troponin T levels correlated closely with creatine kinase MB enzyme activity in blood serum. In all, this immunoassay was superior to other biomarkers of acute myocardial infarction (aspartate aminotransferase, lactate dehydrogenase, and creatine kinase MB). However, it had an essential drawback: many cross-interactions (nonspecific or false positives) of diagnostic antibodies with the skeletal troponins. This disadvantage resulted in frequent false-positive results in the presence of skeletal muscle damage and/or disease.
In second-generation troponin determination methods, specificity increased. Further improvement in third and fourthgeneration immunoassays permitted complete discrimination of nonspecific responses, and the minimum defined concentration was reduced. This advancement led to an earlier diagnosis of acute myocardial infarction within 6 to 8 hours from patient admission with chest pain. Therefore, troponin I and troponin T became the new gold standard in diagnosing acute myocardial infarction, which was finally documented in 2000 by leading experts in the European and American cardiological communities [35].
However, the time required for laboratory confirmation of ischemic cardiomyocyte necrosis remained relatively large and inadequate for an early diagnosis. For this reason, further work continued to find new biomarkers and improve the sensitivity of troponin determination methods [36]. Thus, between 2007 and 2010, highly sensitive methods for determining troponins (high-sensitivity cardiac troponins I and T) – also called fifthgeneration immunoassays – emerged. The minimum detectable concentration from immunochemical methods was only 1 to 10 nanograms per liter, which was tens and hundreds of times less than some of the moderately sensitive methods used up to that time, and thousands of times less than the prototypes created more than 35 years ago. The time spent in the laboratory study with highly sensitive immunoassays was only 20 to 30 minutes [37].
Currently, there are many diagnostic immunoassays on the market designed to detect troponin I and troponin T. They all differ in their analytical characteristics, and the laboratory results obtained when using the same serum often do not coincide. Standardizing different methods for defining troponin I and troponin T is essential [38]. Suppose a patient needs to be transferred to another hospital that uses a different method to determine troponin. In that case, the results cannot be compared to identify the elevation kinetics of acute myocardial infarction. Therefore, repeated studies are needed, requiring additional time and money.
The International Federation of Clinical Chemistry conducts independent expert evaluations and systematizes data on cardiac troponin determination methods. According to this organization, RocheDiagnostics, Abbot, Beckman Coulter, Ortho, Siemens, Singulex, bioMerieux, and LSI Medience produce the most reliable and high-quality highly sensitive immunoassays. Roche Diagnostics only manufactures kits for the determination of high-sensitivity cardiac troponin T. In contrast, the other companies listed above manufacture diagnostic kits to determine high-sensitivity cardiac troponin I, so the standardization problem mainly concerns the latter.
High-sensitivity cardiac troponin I levels may differ if obtained by methods from different manufacturers. One of the main reasons for mismatched results is using different diagnostic antibodies that target different epitopes of troponin I and troponin T molecules. In acute myocardial infarction, many fragments of troponin molecules circulate in the serum, which have different stability and half-life. When antibodies against unstable or more stable epitopes of troponin molecules are used, the result may be underestimated or overestimated, respectively. Troponin breakdown and clearance are poorly studied but are likely to occur continuously and depend on many factors, including drug prescription. In addition, some antigenic epitopes of troponin I and troponin T molecules may be targeted by autoantibodies and heterophile antibodies resulting in falsepositive and false-negative results. Further research on troponin molecules breakdown and elimination and the influence of autoantibodies and heterophile antibodies is needed to improve troponin immunoassays' quality.
To date, many healthcare institutions are using highly sensitive troponin test systems. A Anand and colleagues recently conducted a global study to assess the Universal Definition of Myocardial Infarction [1] recommendations on highly sensitive troponins, using a specially designed telephone questionnaire format. The authors interviewed physicians at 1902 medical centers in 23 countries and five continents. Cardiac troponins were used as the primary diagnostic marker for acute myocardial infarction in 96% of the centers, and creatine kinase MB continues to be used in some Latin American countries (Argentina, Mexico). Only 41% of the centers used highsensitivity assays, ranging from 7% in North America to 60% in Europe. Among institutions that used highly sensitive assay methods, the serial measurement strategy with a predominance of accelerated diagnostic pathways (0 to 3 hours) was used more frequently, with the 99th percentile diagnostic threshold most often taken into account. However, only 18% of the centers adjusted the 99th percentile thresholds by sex characteristics [39].
Highly sensitive methods for troponin determination: their analytical characteristics, criteria, and classification
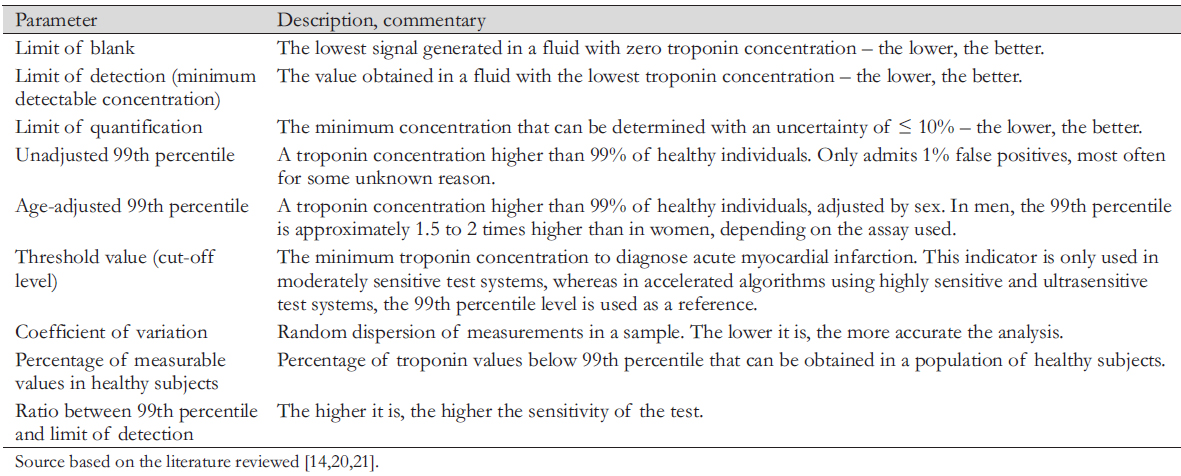
The main analytical characteristics of the quality of troponin immunoassays are the limit of blank ─ the lowest signal generated in fluid with zero troponin concentration; the limit of detection ─ the minimum detectable concentration; the limit of quantification (limit of quantitative determination); unadjusted and age-adjusted 99th percentile; percentage of measurable values in healthy individuals; coefficient of variation; and the ratio of 99th percentile and the detection limit (Table 2) [21],[40],[41].
Many investigators are concerned about which assay should qualify as highly sensitive. The International Federation of Clinical Chemistry Working Group of the Committee on Clinical Application of Cardiac Biomarkers has proposed two criteria for a method to be designated as highly sensitive [21]. First, the percentage coefficient of variation in establishing the 99th percentile values should not exceed 10%. Second, in more than 50% of healthy individuals, the troponin concentration should be above the detection limit of the analytical method. However, many of the methods designated as highly sensitive do not meet these criteria. For highly sensitive assays, all journals, manufacturers, laboratories, and institutions should measure in nanograms per liter to avoid confusion and decimal points followed by unnecessary zeros used in moderately sensitive and some modern highly sensitive assays [21].
The clinical and diagnostic value of high-sensitivity cardiac troponin T and I results is directly related to the analytical characteristics of the diagnostics method used (Table 2). The experts' recommendations of the International Federation of Clinical Chemistry should be followed to establish the analytical parameters of the test system. So, for example, to establish the 99th percentile values according to sex, it is necessary to determine troponin in at least 300 women and 300 men.
 Full size
Full size Subsequently, these values can be adjusted when new data are received. Ideally, each laboratory should establish its own 99th percentile, which would correspond to the test system and analyzer used and the characteristics of the given population. However, given the complexity and cost of such studies, it is permissible to focus on parameters provided by the manufacturers [21],[42].
Establishing optimal values for the 99th percentile is crucial and involves several key questions: how should reference groups be selected? What statistical calculation method should be applied? The definition of a healthy person is also a matter of discussion [21]. How should patients be selected? Should we select young (under 30 years) or those who coincide with patients with classic acute myocardial infarction (40 to 90 years)? What criteria should be used to designate "healthy" patients? Should we use a simple survey (questionnaire) or a complete medical examination – including both physical and instrumental laboratory studies (electrocardiography, echocardiography, determination of natriuretic peptide concentration, creatinine level)? The latter option is ideal but expensive.
Selection of the control group according to strict criteria shifts the 99th percentile to lower values [21,42,43]. Furthermore, it is necessary to have a unified statistical approach when calculating the 99th percentile. The nonparametric method (Harrell-Davis method) and the robust (stable) statistical method give different values for the 99th percentile. Thus, the aforementioned conditions explain the significant variation of the 99th percentile calculated by the different manufacturers.
On the other hand, some new rapid diagnostic algorithms (oneand two-hour) do not focus on the 99th percentile level as the reference diagnostic threshold. Instead, they use lower cut-off values to decide hospitalization and invasive interventions. This approach is appealing because many patients with highsensitivity cardiac troponin concentrations ranging from the limit of detection (or limit of quantification) to the 99th percentile have a higher risk of adverse outcomes compared with those with minimal or undetectable values (i.e., below the limit of detection or limit of quantification). The benefit of these strategies has been demonstrated in several studies to rapidly exclude acute coronary syndrome and identify patients at elevated risk of adverse cardiovascular events at 30 days [44],[45],[46],[47],[48].
The limit of detection is essential to diagnose acute myocardial infarction early in time. The firstand second-generation immunoassay methods had a detection limit between 100 to 500 nanograms per liter. Therefore, acute myocardial infarction was diagnosed too late (after 12 to 24 hours). In some cases, smallfocal infarctions were missed, and no troponin was detected in any healthy patient (0% of the values measured in the reference population).
With current highly sensitive assays, the detection limit may be only a few nanograms per liter and even less than 1 nanogram per liter, which is hundreds of times more sensitive than previous assays, allowing the detection of myocardial damage almost at a cellular level. The percentage of measured troponin values ranges from 50 to 100% [21]. Garcia-Osuna et al. recently studied the analytical characteristics of a new method that detects troponin I at a molecule level. The study showed that this method is approximately 10 times more sensitive than the currently used high-sensitivity cardiac troponin I methods. The detection limit was 0.08 to 0.12 nanograms per liter, and the proportion of healthy subjects with measurable troponin concentrations reached 99.5%. Healthy subjects were strictly selected (based on their anamnesis and normal natriuretic peptide and creatinine levels). The median of high-sensitivity cardiac troponin I was significantly higher in men than women and in the elderly compared with the young. This finding indicates the need to reflect age-related characteristics in high-sensitivity cardiac troponin I levels. This hypersensitive immunoassay is significantly superior to other existing highly sensitive methods [49]. This sensitivity was achieved using four types of antibodies: two of them are directed to epitopes located at the center of troponin and two to epitopes located at both ends of the molecule. Such a test provides a higher uptake of the troponin I molecule and its fragments than test systems based on the use of two or three types of antibodies.
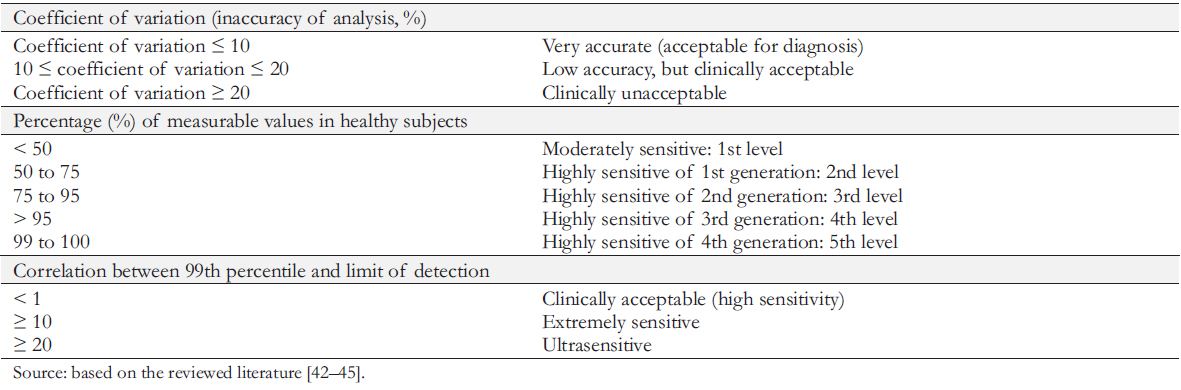
The parameter that determines the accuracy of the immunoassay is the coefficient of variation. The method is considered highly accurate and meets the International Federation of Clinical Chemistry requirements when serially determining the troponin level in a sample, the average dispersion of the results does not exceed 10% (coefficient of variation less than or equal to 10%).
However, due to the limited commercial availability of high precision tests, troponin tests with coefficients of variation between 10 and 20% are still widely used in many laboratories. These test systems can lead to false-positive and false-negative results. Tests with a coefficient of variation greater than 20% are unacceptable for clinical use and should be excluded (Table 3). A significant improvement in the analytical parameters of highly sensitive tests introduced an additional classification of "functional" methods based on the relationship between the 99th percentile and the limit of detection. The higher the 99th percentile/detection limit correlation, the higher the probability of identifying subjects with measurable values.
Table 4 summarizes some of the highly sensitive modern test systems available for clinical use, as well as their analytical parameters (according to data from International Federation of Clinical Chemistry, 2020) [50].
 Full size
Full size 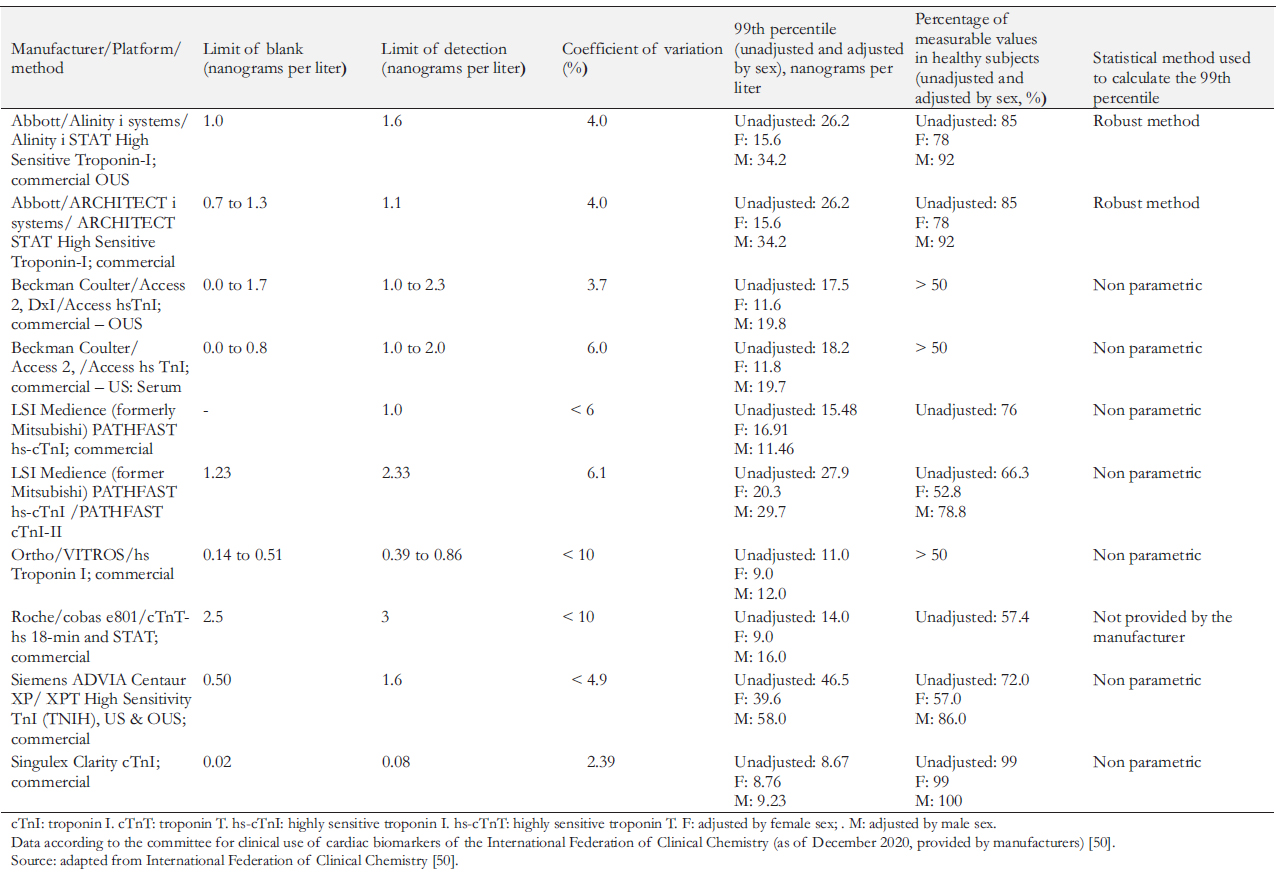 Full size
Full size Conclusion
Laboratory methods for detecting cardiac troponins remain an essential tool to diagnose acute myocardial infarction. Our changing knowledge on cardiac troponins biochemistry continuously improves determination methods and opens new possibilities for their use in laboratory diagnostics.
Cardiac troponins are a specific indicator of myocardial damage, regardless of etiology. In the future, they may be used – not only in cardiology – but also in other areas of medicine.
It is crucial to understand that troponins results depend on their determination methods and their main analytical characteristics, such as unadjusted and sex-adjusted 99th percentile, the limit of blank, minimum defined concentration, and coefficient of variation, among others. This knowledge is needed to use cardiac troponins as a diagnostic tool effectively.
Further work is needed to reveal the mechanisms and effects of age and daytime on highly sensitive troponin levels. In addition, promising new opportunities to investigate cardiac troponin molecules in noninvasive body fluids should be evaluated.
Notes
Contributor roles
AMCh: conceptualization, methodology, software, validation, formal analysis, research, resources, data curation, writing, first draft, revision, editing.
Competing interests
The author completed the ICMJE conflict of interest statement and declared that he/she received no funding for the completion of this article; has no financial relationships with organizations that may have an interest in the published article in the past three years; and has no other relationships or activities that may influence the publication of the article. The form can be requested by contacting the responsible author or the Editorial Board of the Journal.
Funding
The present work did not receive funding for the preparation of the manuscript.
Ethics
The present article is exempt from evaluation by an ethics committee due to the study’s design, which corresponds to a review of the available literature.
Data sharing statement
The data is available upon request.
Provenance and peer review
Not commissioned Externally peer-reviewed by three reviewers, double blind
Language of submission
Spanish.

